Aya Sakaya, Folium Labs
Download the article in PDF format
Introduction
Cannabinoids commonly refer to compounds extracted from, among other cannabis plants, Cannabis sativa and Cannabis indica. The most studied and researched cannabinoids are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) thanks to their more pronounced psychedelic and therapeutic activity. With increasing research on the therapeutic benefits of cannabinoids, accompanied by increased rate of legalization, the medicinal cannabis industry has grown significantly and is estimated to reach over 33 billion dollars in the United States by 2025.
Knowledge of the therapeutic and medicinal activity of cannabinoids dates back to 4000 B.C.1 CBD and THC have been linked to a wide range of therapeutic activity and potential, such as treatment of pain, inflammation, asthma, sleep disorders, anorexia, migraines, and symptoms of multiple sclerosis and schizophrenia, among other conditions.1, 2
While cannabinoids present with a large therapeutic activity and potential, their medical remain limited due to the low stability, solubility, absorption, and short half life of conventional cannabis formulations.3 Cannabinoids are lipophilic compounds that are poorly water soluble in water, which limits their ability to penetrate and be absorbed by cells or enter blood circulation, resulting in their poor bioavailability. They also demonstrate poor stability, as they are easy to degrade upon exposure to light or temperature or via auto-oxidation.4 Medicinal cannabis products need to also provide standard and consistent doses, which is difficult to achieve through common route of administration such when inhaled (smoking, vaping) or taken orally. As such, there has been a lot of focus on developing novel drug delivery technologies that enhance the bioavailability and stability of cannabis products.
Routes of administration
The pharmacokinetic (PK) and pharmacodynamic (PD) parameters of cannabis products will depend heavily on how efficiently the administered dose reaches and is absorbed by its target site. This means that careful consideration must be given to product formulation and administration route.
While intravenous injections might be the most efficient route of administration in terms of all the administered dose reaching the bloodstream with fast onset of action, it remains the least preferred route of administration due to its invasive nature. In contrast, a survey taken by 953 participants shows that the pulmonary route of administration, i.e. by smoking or vaping, is by far the preferred route of administration of cannabis products.2, 5 The pulmonary route allows bypassing the first-pass metabolism which is responsible of clearing a large percentage of an administered dose of a drug, and the CBD/THC is transported immediately to the central nervous system, resulting in a very high bioavailability and a fast onset of action. However, the pulmonary route of administration suffers the main drawback of inconsistent dosage, as the bioavailability will strongly depend on the user’s inhalation technique and smoking history. For example, the bioavailability of smoked cannabis can range between 2% and 56%.6 Another major drawback of smoked cannabis, and perhaps the most obvious one, is the inhalation of ~2000 toxic and carcinogenic compounds.
Transmucosal administration is another popular route that avoid the first-pass metabolism. It involves absorption of cannabis product directly across a mucous membrane and into the bloodstream. Examples include products intended to be dissolved inside the cheek (buccal), under the tongue (sublingual), or sprayed into the nose (intranasal). Transmucosal administration is non- invasive and allows for improved bioavailability, rapid absorption, and fast onset of action when compared to orally administered cannabis products.
The transdermal route of administration (e.g. through topical creams, patches, etc…) also constitutes an easy to use method than allows the targeting of localized pain or inflammation, such as the case of arthritis. The transdermal route also bypasses the first-pass metabolism, which improves the bioavailability of administered drugs.1 It also allows the prolonged, steady infusion of a drug over multiple days such as in the case of topical patches, which improves patient adherence. This method however is limited by the small dose that can be administered at a time, as well as the ability of the cannabis compounds to cross the skin barrier. While employment of penetration enhancers might alleviate those limitations, their use exacerbates the already present chance of skin irritation at the application site.1, 2
Oral administration of medicinal cannabis product is the most commonly used administration route.2 Its biggest advantages are its ease of use and patient compliance,7 and the ability to control the administered dose compared to pulmonary or transdermal administration.1 While the administered dose can be controlled with great precision, the PK profile of orally administered cannabis products shows large variability in terms of both peak concentration and time to reach peak concentration. For example, the peak plasma concentration of orally administered Dronabinol, an FDA approve synthetic THC product, can range between 2.1 and 16.9 ng/mL, while also showing a long a variable time to reach this peak plasma concentration ranging from 1 h to 8 h.2, 6 Such large variabilities in the PK parameters of orally administered cannabis products can strongly effect their therapeutic potential from patient to patient.6 Another main complication of orally administered cannabis products, besides their low absorbance by cells, is the clearance of the vast majority of the administered dose by first-pass metabolism. The multiple challenges faced through oral administration of cannabis products has led to resorting to novel formulations within drug carrier delivery systems to improve their bioavailability and PK profile.
Current carrier technologies for the oral delivery of cannabinoids
Encapsulation of lipophilic compounds within nanocarriers provides improved delivery and bioavailability enabling the administration of lower and less frequent doses.2 It also acts as a protection layer against the harsh acidic environment in the digestive track, and enables to target the delivery of active ingredients to specific sites of interest.1, 2 Formulations within nanocarriers can also be combined with penetration enhancers or secondary non-cannabinoid compounds extracted from C. sativa that have been shown to enhance the therapeutic effects of cannabinoids through what is known as the entourage effect.2, 8 A wide range of nanocarrier technologies have been employed for the enhanced delivery of cannabis compounds. Ascension Sciences for example developsa range of both lipid-based and polymer-based nanoformulations platforms and technologies for the enhanced delivery of cannabinoids.
Liposomes are small vesicles composed of a phospholipid bilayer (thin double layer of lipids) with and entrapped water pool inside the vesicle. They are very attractive thanks to their biocompatibility, stability (months), and protection against oxidation and other environmental stressors in the gastrointestinal (GI) track. QuickSilver scientific for example claims to increase the uptake time of CBD by 5.3 times when they encapsulate it within liposomes, The main drawback of liposomes however in that they can only entrap lipophilic compounds like CBD and THC within their thin lipid membrane while the vast majority of their volume is aqueous and thus inaccessible, which largely limits the dose they can carry.
Lipid-based micelles avoid that problem, as their entire core is used to entrap poorly water-soluble compounds. They are also much simpler to form than liposomes. Glow LifeTech employs their proprietary MyCellTM micellization technology to create water-compatible liquid concentrates of broad spectrum or isolated cannabinoids such as THC and CBD. However, micelles have shorter stability that liposomes, and are mainly composed on surfactants, which are known to cause irritation and low patient tolerability.
Another commonly used lipid-based carrier systems are lipid-based emulsions. Companies like SōRSE Technology and Caliper Foods utilized proprietary emulsification technologies to create stable, water soluble CBD solutions, which they claim to improve CBD bioavailability (30× in the first 30 minutes in the case of Caliper Foods). Industrial Sonomechanics uses their patented Barbell Horn® Ultrasonic Technology (BHUT) to create stable CBD nanoemulsions. Vertosa, Inc. on the other hand creates custom-made cannabinoid emulsions and infused products depending on the consumer’s preferences. Self micro-/nano-emulsifying drug delivery systems (SMEDDS/SNEDDS) have gain particular interest and are currently being employed in a large number of early-stage product development. Self-emulsifying delivery systems are a stale, solid mixture of lipid/oil, surfactants and co-surfactants, and active ingredient, which spontaneously form small, nano-sized emulsions upon interaction with biological fluid such as upon ingestion. EmbarkNano uses a wide rage of formulations for the oral and topical delivery of CBD through their μGOO, μSHOT, μCREAM and μMIX nanoemulsion-based formulations. On the other hand, Micellae Delivery Systems were able to reduce the time to reach maximum CBD concentration from 4-6 hours to 20-30 minutes when formulationg CBD using their O2W SMEDDS technology. One of the main drawbacks of micro- and nano-emulsions is the need of a large concentration of surfactants to ensure the formation of small-sized emulsions. Large concentrations of surfactants affect however the ability of those emulsions to contain lipophilic compounds upon their hydration and have been linked to low patient tolerability as mentioned above.
Lexaria Bioscience uses their patented DehydraTECHTM formulation and processing technology to chemically link individual cannabinoid and fatty acid (lipid) molecules infused within a food substrate (such as cocoa powder, tapioca, and gum arabic). They claim their formulation allows to enhance the taste, smell, intestinal tissue permeability, and overall bioavailability of cannabinoid compounds.
Polymeric nanoparticles, like lipid-based carriers, are able to encapsulate lipophilic compounds with high efficiency, while also having better stability. They are often made of biocompatible and biodegradable polymers like poly (lactic-co-glycolic acid) (PLGA).9 They are often formulated with polyethylene glycol (PEG) for added water solubility and stability, as PEG greatly decreases nanoparticle detection and clearance by the reticuloendothelial system, thus increasing drug circulation time. Polymeric nanoparticles can also be functionalized to be stimuli-responsive, i.e., release their drug load upon encountering a specific signal such as a pH or temperature change of the presence of a specific enzyme that leads to polymer degradation and drug release.10
Another polymer-based carrier approach that doesn’t rely on the formation of nanoparticles is the use of solid dispersions, where lipophilic compounds are dispersed within a solid, often a water-soluble polymeric matrix. This method can greatly enhance the bioavailability of poorly water-soluble compounds such as CBD and THC by decreasing their particle size and increasing their surface area.11 Nabilone for example, commercially known as CesametTM by Bausch Health Co., consists of a synthetic THC derivative formulated within a solid polyvinyl pyrrolidone (PVP) matrix, has a very high bioavailability, reaching up to 95%.1
A particular subcategory of solid dispersions is the formation of inclusion complexes, where lipophilic “guest” compounds are encapsulated on a molecular level (i.e., separate molecules) within the cavities of a water-soluble “host”. Azuca Thermodynamic Individual Molecular Encapsulation (TiME InfusionTM) technology creates a thermodynamic matric of individually encapsulated THC molecules with improved water-solubility. Cyclodextrins (CD) and their synthetic derivatives are the most frequently used “host” compounds. While CD inclusion complexes have the potential to greatly improve the bioavailability of lipophilic drugs, formation of these inclusion complexes is very energy exhaustive and their preparation extends over several days.12, 13
Folium Labs CBD and THC delivery technology
Folium Labs focuses on the development of novel drug delivery systems for the enhanced delivery and bioavailability of lipophilic natural pharmaceuticals (nutraceuticals). We utilize a proprietary solid-state reactive mixing (SSRM) technology to encapsulate lipophilic nutraceuticals within hyaluronic acid (HA) matrices to form highly absorbable complexes.
Scheme 1. SSRM technology is based on the complexation of active ingredients within a hyaluronic acid matrix to provide molecular complexes with improved PK profiles.
HA is a natural, biocompatible, and biodegradable polymer with excellent mucoadhesive properties, making it an excellent carrier for transmucosal administration.14 HA has specific receptors expressed on cell surfaces that promote it adsorption into cells through endocytosis. These receptors are present in large concentrations joint tissue and fluid,15 and are overexpressed by tumors,16 making HA an ideal carrier to deliver anti-inflammatories and chemotherapeutics with little to no synthetic alteration required for targeted delivery.
Our SSRM technology consists of a one-step, low energy, and solvent-free “green” complexation process in which active ingredients get incorporated within HA through hydrogen bonding and van der Waals interactions. SSRM is a low cost and highly scalable method that does not depend on specific drug-HA interactions, making it easily translatable to a wide range of nutraceuticals and other therapeutic compounds. As SSRM utilizes commercially available, naturally sourced ingredients, it is easy and fast to meet regulatory compliance and bring products to market.
Proof of concept animal studies were conducted on male Sprague Dawley rats in order to assess the ability of our SSRM technology to enhance the bioavailability of orally administered THC and CBD. The results are shown in Figure 1 below. Doses were administered orally to a concentration of 20 mg/kg of CBD or THC, formulated via SSRM technology or in their pure form in coconut oil.
HA-CBD complex showed an enhanced bioavailability (peak concentration) and faster onset (time to peak concentration) compared to pure CBD. We recorded an 8-fold and a 5.4-fold increase in peak concentration and area under curve (AUC), respectively, when CBD was administered within an HA complex.
Data recorded with THC was even more remarkable, as the bioavailability of pure THC under the administered dose was too low to be quantified, whereas THC administered within the SSRM HA matrix exhibited a large peak concentration of 61.2 ng/mL, reached after 2 hours of administration. These PK parameters are on par with those detected through pulmonary administration, and much better (higher concentration and faster onset) than the parameters observed through oral administration of pure or synthetic THC for similar administered doses.2, 17, 18 As the concentration of pure THC was too low to quantify, we were not able to obtain a quantitative percent-improvement of bioavailability, however it is safe to conclude that our SSRM technology is clearly able to immensely enhance the bioavailability of THC.
It is worth highlighting that the data presented in Figure 1 is only preliminary, and no optimization steps were taken to evaluate the full potential of the SSRM. We believe that with correct optimizations, we can achieve even more impressed enhancements in the bioavailability of CBD and THC than what is already presented.
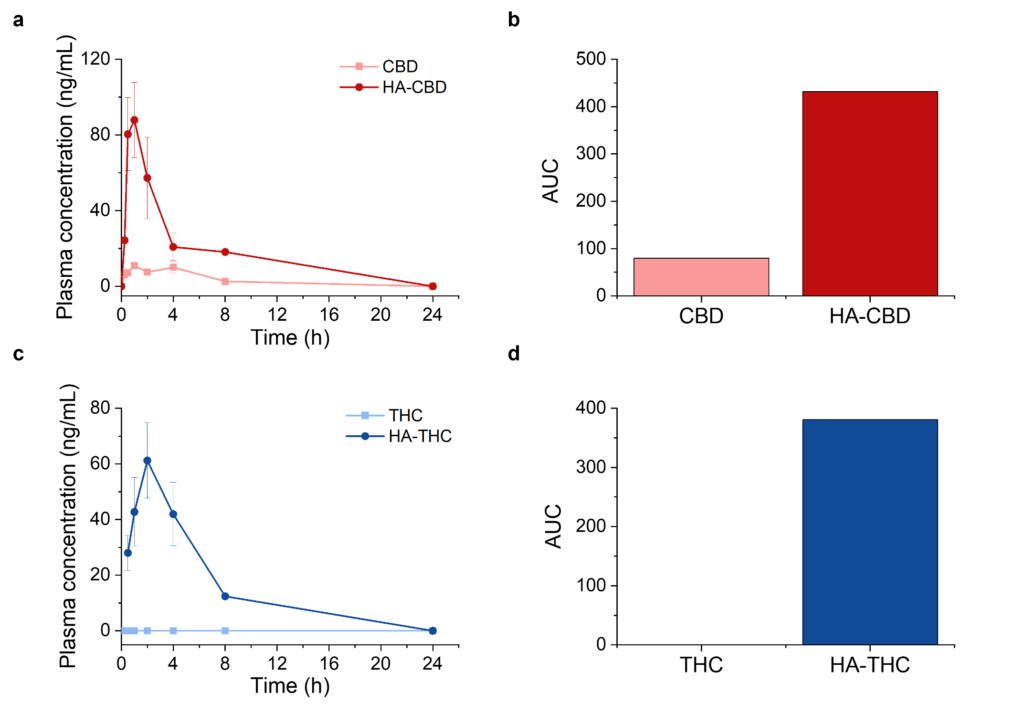
Figure 1. Pharmacokinetics data obtained when administering pure CBD or THC vs an HA-CBD or HA-THC complex obtained through SSRM technology. (a) Plasma concentration over time recorded for pure CBD vs HA-CBD. (b) Area under curve (AUC) obtained from traces shown in panel (a). (c) Plasma concentration over time recorded for pure THC vs HA-THC. (b) Area under curve (AUC) obtained from traces shown in panel (c). Administered dose of CBD or THC was 20 mg/kg. Data corresponds to mean ± standard error of mean (SEM).
References
1. Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F., Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23 (10), 2478.
2. Kebede, L.; Masoomi Dezfooli, S.; Seyfoddin, A., Medicinal Cannabis Pharmacokinetics and Potential Methods of Delivery. Pharmaceutical Development and Technology 2022, (just-accepted), 1-49.
3. Rani, K.; Paliwal, S., A review on targeted drug delivery: Its entire focus on advanced therapeutics and diagnostics. Sch. J. App. Med. Sci 2014, 2 (1C), 328-31.
4. Lindholst, C., Long term stability of cannabis resin and cannabis extracts. Australian Journal of Forensic Sciences 2010, 42 (3), 181-190.
5. Eisenberg, E.; Ogintz, M.; Almog, S., The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study. Journal of pain & palliative care pharmacotherapy 2014, 28 (3), 216-225.
6. Huestis, M. A., Human cannabinoid pharmacokinetics. Chemistry & biodiversity 2007, 4 (8), 1770.
7. Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A. J.; Hoffman, A., Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. Journal of Controlled Release 2017, 266, 1-7.
8. Bonn-Miller, M. O.; ElSohly, M. A.; Loflin, M. J.; Chandra, S.; Vandrey, R., Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. International Review of Psychiatry 2018, 30 (3), 277-284.
9. Rout, G. K.; Shin, H.-S.; Gouda, S.; Sahoo, S.; Das, G.; Fraceto, L. F.; Patra, J. K., Current advances in nanocarriers for biomedical research and their applications. Artificial Cells, Nanomedicine, and Biotechnology 2018, 46 (sup2), 1053-1062.
10. Liechty, W. B.; Kryscio, D. R.; Slaughter, B. V.; Peppas, N. A., Polymers for drug delivery systems. Annual review of chemical and biomolecular engineering 2010, 1, 149-173.
11. Dalvi, P.; Gerange, A.; Ingale, P., Solid dispersion: strategy to enhance solubility. Journal of Drug Delivery and Therapeutics 2015, 5 (2), 20-28.
12. Pang, L.; Zhu, S.; Ma, J.; Zhu, L.; Liu, Y.; Ou, G.; Li, R.; Wang, Y.; Liang, Y.; Jin, X., Intranasal temperature-sensitive hydrogels of cannabidiol inclusion complex for the treatment of post-traumatic stress disorder. Acta Pharmaceutica Sinica B 2021, 11 (7), 2031-2047.
13. Li, H.; Chang, S.-L.; Chang, T.-R.; You, Y.; Wang, X.-D.; Wang, L.-W.; Yuan, X.-F.; Tan, M.-H.; Wang, P.-D.; Xu, P.-W., Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. Journal of Molecular Liquids 2021, 334, 116070.
14. Guo, Y.-g.; Singh, A. P., Emerging strategies for enhancing buccal and sublingual administration of nutraceuticals and pharamaceuticals. Journal of Drug Delivery Science and Technology 2019, 52, 440-451.
15. Huang, G.; Huang, H., Application of hyaluronic acid as carriers in drug delivery. Drug delivery 2018, 25 (1), 766-772.
16. Cho, H.-J., Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. Journal of Pharmaceutical Investigation 2020, 50 (2), 115-129.
17. Huestis, M. A.; Henningfield, J. E.; Cone, E. J., Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. Journal of analytical Toxicology 1992, 16 (5), 276-282.
18. Kim, K.-R.; Yoon, H.-R., Rapid screening for acidic non-steroidal anti-inflammatory drugs in urine by gas chromatography-mass spectrometry in the selected-ion monitoring mode. Journal of Chromatography B: Biomedical Sciences and Applications 1996, 682 (1), 55-66.

