Aya Sakaya, Folium Labs
Download the article in PDF format
Introduction
Drug delivery technologies are platforms that enable the conversion of potential therapeutics into successful therapies. These biotechnologies have allowed to improve patient health through development of novel drugs and pharmaceutical products. Nutraceuticals in particular have gained an increased public and scientific attention. Nutraceuticals are naturally occurring nutrients or food ingredients that have been shown to have medical and heath benefits in disease prevention and treatment.1 Nutraceuticals have seen increased interest and market size since, besides their health benefits, they are relatively more biocompatible and cheaper to market that synthetic drugs, and show a higher public acceptance as the general public is starting to show increased interest in and consciousness of healthy living. A report by Statista Estimates, Grand View Research indicates that the US nutraceutical market was worth over 71 billion dollars in 2017 and is estimated to reach over 133 billion dollars by 2025.
Despite their vast potential benefit, a large number of nutraceuticals are hydrophobic (poorly water soluble), resulting in poor bioavailability when taken in their “pure” form. This challenge is not limited to the nutraceutical industry, as it is estimated that 90% of preclinical small molecule drug candidates are poorly water soluble.2 While an accurate definition of bioavailability defers slightly between different sources and fields, it generally refers to the extent to which an administered drug, nutrient, or substance is absorbed and used by its intended target in the body. It is thus essential to maximize the bioavailability of any pharmaceutical or nutraceutical of interest to ensure maximum benefit and efficacy. While increasing the administered dose in order to increase the amount of absorbed drug to a therapeutic concentration might seem like a simple solution, this approach is not recommended as it involves numerous complications in terms of the feasibility of administering such a large dose, potential side effects such as toxicity and immunogenicity, and the monetary and environmental cost of wasted material.
Numerous approaches have been taken to improve the bioavailability of insoluble drugs and nutrients.2, 3 These approaches generally fall under three strategies: modifications of the chemical or physical properties of the drug; mixing with co-ingredients or co-solvents; and development of drug carrier systems that improve the interactions between the drug and surrounding biological molecules, tissues and cells. Current drug delivery biotechnologies often combine elements of some or all these three strategies with the aim to improve drug solubility, circulation time, penetration and absorbance, targeted activity, off-target accumulation, and patient tolerability and acceptance.
Drug modification
Drug modification includes chemical modifications to the structure of the drug itself and/or changes to its physical properties with the aim to improve the interaction between the drug and its microenvironment, mainly by improving its water solubility.
The solubility and absorption of a drug can be greatly improved by chemically altering the existing functional groups of the drug. For example, ritonavir (Norvir by Abbvie, Inc), a critical protease inhibitor for HIV, is modified by a thiazole moieties to improve it’s water solubility and metabolic stability.4 Another approach is the synthesis of prodrugs, most commonly alkyl ester prodrugs such as the angiotensin-converting-enzyme inhibitors benazepril (Lotensin by Novartis Pharmaceuticals, Inc) and enalapril (Vasotec by Merck).5 The ester prodrugs of improved solubility and penetration are formulated as non-active precursors which undergo in vivo chemical or enzymatic cleavage of the ester group upon there uptake and generate the active drug. While chemical modifications can serve as a versatile tool, it can be extremely challenging and resource exhausting to achieve. Chemical modifications might include challenging synthetic routes, where the product (if obtained) must go extensive pre-clinical and clinical characterization and testing to ensure the desired therapeutic and chemical properties are obtained. This means that any new product will be subjected to extensive regulatory considerations.
Modifications to the physical properties of the drug may circumvent the above-mentioned synthetic and regulatory challenges of chemical modifications. “Physical” modifications primarily target the crystal form of the drug. When a drug possesses a stable crystal form, it can be very challenging to break those strong intermolecular interactions in order to solubilize it. Disordered, amorphous packing on the other hand renders the drug more water soluble.6 One case example is Ceftin by GSK, an amorphous form of the antibiotic cefuroxime which is completely insoluble in water in its original form. Because the solubility of amorphous forms relies on the reduces molecular-packing stability of the latter, they can be very challenging to form and stabilize, and thus are often protected by multiple patents which prevent early generic entry.
Co-ingredients and co-solvents
Another strategy to improve the bioavailability of insoluble drugs and nutraceuticals is by addition of a co-ingredient or co-solvent that improve its solubility and penetration. One of the main co-ingredient strategies is the addition of pH modifiers, which can alter the pH of the drug’s local environment into one in which the drug is in an ionic and more soluble form.7 A classic example is the antibiotic Ciprofloxacin (Cipro IV), which is poorly soluble in water alone, but is formulated with hydrochloric acid and lactic acid for improved solubility.8 This approach however relies on the ionizability (acidity) of the drug, and the compatibility between it’s optimal solubility pH range and physiological pH.
The bioavailability of nutraceuticals can be further improved by addition of excipients (co-ingredients) that act as penetration enhancers. In fact, many penetration enhancers can be found in natural sources, and are often plant extracts, alkaloids and flavonoids such as piperine and quercitin, with well studied mechanisms of action.9 BioPerine® is a patented standardized piperine extract by Sabinsa Corporation that has been shown to increase the bioavailability of certain nutraceuticals. Other natural ingredients, such as Piper nigrum L.), long pepper (Piper longum L.) and ginger (Zingiber officinalis L. have also been shown to increase the bioavailability of drugs, though their mechanism of action is not well know.9 Non-herbal penetration enhancers include fatty acids, alcohols and cholates, which often decrease the barrier effect of cells by disrupting the lipid arrangements between them.10 Zemvelo by Mineralife Nutraceuticals relies on the addition of fulvic acid in its formulations to promote the absorption and bioavailability of delivered nutrients and minerals.
Formulation of drugs with co-solvents is also a commonly used practice. It involves increasing drug solubility and penetration by addition of co-solvents such as alcohol, polymers, and biomolecules that solubilize non-polar drugs, but require the addition of surfactants to prevent precipitation upon dilution in biological fluid. Chemotherapy drugs paclitaxel and docetaxel have been commonly formulated using the co-solvent approach, their most common formulations being Taxol (by Bristol-Myers Squibb) and Taxotere (Sanofi-Aventis), respectively. Both these formulations however lead to hypersensitivity reactions from the surfactants used. As a result, newer co-solvent formulations use polyethylene glycol (PEG) as co-solvent and Tween 80 as surfactant, which have reported better patient tolerance and less adverse effects compared to the solvents and surfactants used in older formulations. However, the co-solvency method still presents some disadvantages, such as uncontrolled precipitation of the drug upon its interaction with biological fluid, and potential decrease in the stability and effectiveness of the drug.2
Drug carrier systems
Development of drug carrier systems is the most recurrent strategy to improve the bioavailability of insoluble drugs (fig. 1).
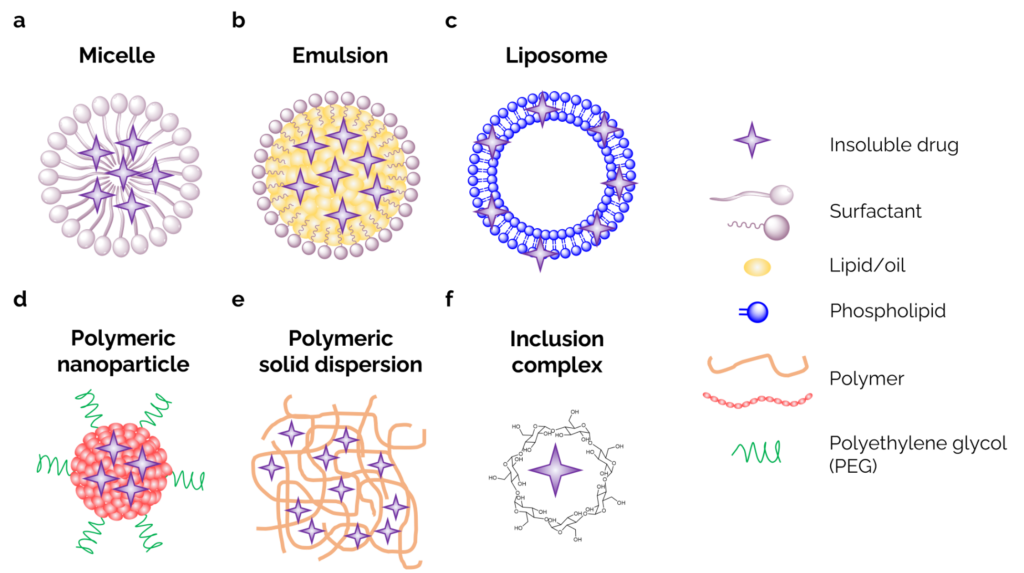
Figure 1. Drug carrier systems of poorly water-soluble drugs. (a) lipid or surfactant-based micelles. (b) mico- and nanoemulsions. (c) phospholipid-based liposomes. (d) PEGylated polymeric nanoparticles. (e) polymer-based solid dispersions. (f) cyclodextrin-based inclusion complexes.
Carrier systems serve as an interface that facilitates the interactions between the pharmaceuticals and their microenvironment, mainly by increasing solubility, penetration, circulation time, and offering potential targeting benefits. Glow LifeTech, for example, relies on the formation of micelles through their plant based MyCellTM Technology to increase the water solubility of fat-soluble nutraceuticals (fig. 1a). Most drug carrier technologies fall under one of two categories: lipid-based or polymer-based drug delivery systems.
Lipid-based drug delivery technologies involve the use of emulsions, micelles, liposomes and proliposomes, or solid-lipid nanoparticles to enhance the solubility of drug and deliver it to its target site. Products such as CUREpodsTM and CUREdropsTM from CURE Pharmaceuticals rely on a range of lipid-based drug delivery technologies in their formulation.
Micro- and nano-emulsions have seen increased use in drug delivery technologies. They are composed of a thermodynamically stable mixture of oil/lipid, water, surfactants and co-surfactants, and the drug itself, and form stable heterogenous systems compared to conventional emulsions (fig. 1b). SōRSE Technology, Industrial Sonomechanics, and VESIsorb® for example rely on the formation of stable nanoemulsions for the improves delivery of nutraceuticals like hemp extracts and cannabidiols. Perhaps the most attractive type of micro-emulsions is self-micro-emulsifying drug delivery systems (SMEDDS). Self-emulsifying formulations involve an isotropic mixture of oil/lipid, surfactants and drug (without water) which spontaneously emulsifies upon ingestion and interaction with gastro-intestinal fluid resulting in droplets smaller that 250 nm in size. μGOO by EmbarkNano is marketed as a nanoemulsion “precursor” to be used to generate their other products such as μSHOT nanoemulsions and μCREAM “nano topical”. Lipid based micro-emulsions, such as Vitalipid by Kabi, O2W by Micellae Delivery Systems, and SureNanoTM have been frequently used for the enhanced delivery of nutraceuticals.11-13 As higher surface area of nanoparticles results in their faster absorption, micro-emulsions often require the addition of a large concentration of surfactants in order to reduce their size and increase their surface area-to-volume ratio. This presents the major drawback of self-micro-emulsifying delivery technologies, and emulsion technologies overall, as large concentrations of surfactants may result in a decrease of the drug encapsulation ability of the micro-emulsions due to increase hydrophilicity of the matrix, which may lead to the precipitation of the drug upon dilution in physiological fluid.14 Additionally, use of large concentration of surfactants has been shown to lead to patient hypersensitivity and low tolerance.
Liposomes are a form of lipid-based drug delivery technologies that do not require the use of a surfactant. Liposomes are spherical nanoparticles composed of phospholipid bilayers. They contain an aqueous center that is entrapped by a hydrophobic lipid bilayer shell (fig. 1c). Liposomes can thus be great carriers of both hydrophobic and hydrophilic compounds. Hi-Tech Pharmaceuticals for example use their CyclosomesTM technology to entrap hydrophobic compounds inside cyclodextrins and delivery them using a liposome carrier. Quicksilver liposomal Delivery Systems® by Quicksilver Scientific also rely on high-phosphatidylcholine phospholipid mixes to produce stable small delivery vehicles, whereas Indena S.p.A. relies on natural lecithin phospholipids in there Phytosome® drug delivery system. Liposomes are often formulated with the addition of PEG for additional water solubility and non-immunogenicty, as PEG helps reduce their detection of and clearance. Liposomes can further be functionalized by targeting moieties to provide site-specific activity. Perhaps the most relevant examples of the use of liposomes as carrier systems are the mRNA based COVID 19 vaccines such as the ones developed by Moderna and Pfizer/BioNTech. Moreover, the firs FDA-approved nanoparticle therapeutic was PEGylated liposomal doxorubicin (Doxil by Sequus Pharmaceutical, Inc.). One major drawback of liposomes is their reduced shelf-life and long-term stability, and potential leakage of the entrapped drug over time. A solution to that was the introduction of proliposomes, a dry mixture of powder lipid, drug, and other ingredients that form multilamellar vesicles (liposomes containing liposomes) upon their hydration right before drug administration or upon reaction with physiological fluid in vivo.
Polymer-based drug delivery technologies offer the same advantages as lipid-based technologies (enhanced bioavailability of drugs, site-targeting), with the added benefits of ease of functionalization and higher stability. Polymer-based technologies typically involve liquid formulations of polymeric micelles/nanoparticles, or solid formulations of solid dispersions and co-crystals.
Polymeric micelles are nanoparticles that encapsulate hydrophobic drugs and nutraceuticals as a result of hydrophobic-hydrophilic interactions. To ensure both a hydrophobic environment for the drug as well as solubility in aqueous solutions, the nanoparticles are formed of bi-block of tri-block copolymers, polymers that contain both hydrophobic and hydrophilic blocks (fig. 1d). The polymeric micelles have low critical micellar concentrations, meaning they maintain their stability and form even at low polymer concentrations. They also provide improved solubilization, stability, and patient tolerability compared to micelles formed with hydrophilic surfactants. Genexol-PM by Samyang, a polymeric nanoparticle containing paclitaxel, reported better safety and less side effects compared to its earlier formulations relying of co-solvency with ethanol and cremophore EL. The hydrophobic block of the polymer is often made of PEG, which increases the circulation time of the nanoparticles by making less likely to be cleared by the reticuloendothelial system. However, it has been reported recently that pre-existing anti-PEG antibodies can lead to the quick clearance of PEG-containing nanoparticles, with an increased risk of immunogenicity and adverse events.15, 16
Solid formulations relying on the formations of solid dispersions and co-crystals have been successful at increasing the bioavailability of drugs as well as generating commercial success to the manufacturer.2 Solid dispersions are often formed from a polymer “solvent” such as PEG, polyvinyl pyrrolidone (PVP), or HPMC (a cellulose derivative) with interspersed drug withing the polymer, either in as amorphous form or as a molecular dispersion within the polymer matrix (fig. 1e). Solid dispersions increase the solubility of drugs by preventing the packing of the drug into a stable crystalline form and/or due to an increase in hydrotrophy behavior by forming a molecular adduct.17 Multiple formulations involving solid dispersions have show considerable increase in drug solubility, such as Sporanox byJanssen Pharmaceuticals, a solid dispersion of the antifungal itraconazole in HPMC, or Nimotop by Bayer (Pty) ltd., a solid dispersion of the calcium channel blocker nimodipine in PEG.2 Despite their benefits, solid dispersion are most commonly formed by hot melt extrusion, a very energy exhaustive technique. Other formation techniques include spray-layer, which require the presence of an additional substrate onto which the polymer and drug are deposited.18, 19
One derivative of the solid dispersion method that has seen increased application is the formation of “inclusion complexes”. Inclusion complexes rely on a similar mechanism to molecular solid dispersions in that they form “host-guest” complexes where drugs are held inside matrix cavities by intermolecular interactions such as van der Waals interactions and hydrogen bonding. Inclusion complexes have been formed from natural biomolecules such as fenugreek fibers and starch,20, 21 synthetic cyclic oligomers like calixarenes,22 and poly- and oligosaccharides like arabinogalactan, glycyrrhizin, and cyclodextrins.23 FENUMATTM by Akay Natural Ingredients Private Limited is based on the complexation of active ingredients in fenugreek fiber matrix to enhance the bioavailability of phytonutrients. Natural and synthetic cyclodextrins (CD) are by far the most commonly used “host” molecules in inclusion complexes for drug delivery applications. CDs have a truncated cone structure, with a hollow hydrophobic interior and a hydrophilic exterior, which provides a good environment to trap hydrophobic drugs and solubilize them in aqueous media (fig. 1f). The first FDA approved cyclodextrin-containing drug product was Sopranox oral and IV solution by Janssen Pharmaceuticals, which contained itraconazole entrapped in a synthetic CD.24 Tesseract Medical Research uses their proprietary CyLocTM and DexKeyTM technologies to entrap and then release active ingredients from cyclodextrin cages. These CD inclusion complexes are typically formed by freeze-drying, spray-drying, kneading or co-precipitation.25, 26
Folium Labs drug delivery technology
Here at Folium Labs, we focus on the development of new drug and nutrient delivery platforms. We use a proprietary solid state reactive mixing (SSRM) technology that relies on encapsulating active ingredients and nutrients with hyaluronic acid (HA) matrices.
HA is a naturally occurring polysaccharide, and one of the main components of extracellular matrices. HA is an ideal matrix to form inclusion complexes, as it is biocompatible, biodegradable, naturally occurring in our body, and has excellent mucoadhesive properties that make it an exceptional carrier for buccal and sublingual drug delivery.10 HA and its derivatives have been widely used as carriers of multiple classes of drugs and active molecules (nucleic acids, peptides and proteins, chemotherapeutics, imaging agents, photosensitizers, etc) in multiple drug delivery technologies (nanoparticles, micelles, hydrogels, emulsions, films).27-29 HA has specific receptors expressed on cell surfaces that mediate its absorption into cells (endocytosis). These receptors are present in high concentrations in joint tissue (articular cartilage) and fluid (synovial fluid) and are overexpressed by tumors,27, 28 making HA an ideal carrier to deliver anti-inflammatories and chemotherapeutics with little to no synthetic alteration required for targeted delivery. Moreover, the functional groups (carboxyl and hydroxyl groups) present on HA enable its easy functionalization with bonds that are signal-responsive and break upon encountering specific chemical or physical cues such as pH, light, or molecules that are over expressed in tumor environments, enabling not only targeted tumor delivery, but also triggered release of the drug/nutraceutical of interest.29
Our SSRM technology involves a solvent-free, low energy, green, one-step complexation process. The drugs, nutrients, and matrix used are all commercially available, making SSRM a very low-cost technology. The active ingredients are held within the HA matrix by hydrogen-bonding and van der Waals interactions and do not rely on any specific interactions between the drug and HA, making SSRM an easily scalable and translatable technology to use with a wide range of hydrophobic drugs and nutraceuticals. Moreover, the fact that SSRM is not a nanotechnology, nor does it rely on chemical modifications to the drug or natural HA makes it easier and faster to meet regulatory compliance and bring the products to market.
Proof-of-concept animal studies on a number of nutraceuticals formulated using SSRM have shown a significantly increased bioavailability by up to 10 times depending on the ingredient studies.
References
- DeFelice, S. L., The nutraceutical revolution: its impact on food industry R&D. Trends in Food Science & Technology 1995, 6 (2), 59-61.
2. Kalepu, S.; Nekkanti, V., Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharmaceutica Sinica B 2015, 5 (5), 442-453.
3. Vargason, A. M.; Anselmo, A. C.; Mitragotri, S., The evolution of commercial drug delivery technologies. Nature Biomedical Engineering 2021, 5 (9), 951-967.
4. Kempf, D. J.; Sham, H. L.; Marsh, K. C.; Flentge, C. A.; Betebenner, D.; Green, B. E.; McDonald, E.; Vasavanonda, S.; Saldivar, A.; Wideburg, N. E.; Kati, W. M.; Ruiz, L.; Zhao, C.; Fino, L.; Patterson, J.; Molla, A.; Plattner, J. J.; Norbeck, D. W., Discovery of Ritonavir, a Potent Inhibitor of HIV Protease with High Oral Bioavailability and Clinical Efficacy. Journal of Medicinal Chemistry 1998, 41 (4), 602-617.
5. Beaumont, K.; Webster, R.; Gardner, I.; Dack, K., Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Current Drug Metabolism 2003, 4 (6), 461-485.
6. Laitinen, R.; Löbmann, K.; Strachan, C. J.; Grohganz, H.; Rades, T., Emerging trends in the stabilization of amorphous drugs. International Journal of Pharmaceutics 2013, 453 (1), 65-79.
7. Taniguchi, C.; Kawabata, Y.; Wada, K.; Yamada, S.; Onoue, S., Microenvironmental pH-modification to improve dissolution behavior and oral absorption for drugs with pH-dependent solubility. Expert Opinion on Drug Delivery 2014, 11 (4), 505-516.
8. Breda, S. A.; Jimenez-Kairuz, A. F.; Manzo, R. H.; Olivera, M. E., Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. International Journal of Pharmaceutics 2009, 371 (1), 106-113.
9. Myungjoo, K.; JaeYoul, C.; ByungHo, S.; DukKi, K.; JaehWi, L., Bioavailability enhancing activities of natural compounds from medicinal plants. Journal of Medicinal Plants Research 2009, 3, 1204-1211.
10. Guo, Y.-g.; Pratap Singh, A., Emerging strategies for enhancing buccal and sublingual administration of nutraceuticals and pharamaceuticals. Journal of Drug Delivery Science and Technology 2019, 52, 440-451.
11. Shah, A. V.; Desai, H. H.; Thool, P.; Dalrymple, D.; Serajuddin, A. T. M., Development of self-microemulsifying drug delivery system for oral delivery of poorly water-soluble nutraceuticals. Drug Development and Industrial Pharmacy 2018, 44 (6), 895-901.
12. Tran, T. H.; Guo, Y.; Song, D.; Bruno, R. S.; Lu, X., Quercetin-Containing Self-Nanoemulsifying Drug Delivery System for Improving Oral Bioavailability. Journal of Pharmaceutical Sciences 2014, 103 (3), 840-852.
13. Balakrishnan, P.; Lee, B.-J.; Oh, D. H.; Kim, J. O.; Lee, Y.-I.; Kim, D.-D.; Jee, J.-P.; Lee, Y.-B.; Woo, J. S.; Yong, C. S.; Choi, H.-G., Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. International Journal of Pharmaceutics 2009, 374 (1), 66-72.
14. Pouton, C. W., Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. European Journal of Pharmaceutical Sciences 2000, 11, S93-S98.
15. Wang, X.; Ishida, T.; Kiwada, H., Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. Journal of Controlled Release 2007, 119 (2), 236-244.
16. Povsic, T. J.; Lawrence, M. G.; Lincoff, A. M.; Mehran, R.; Rusconi, C. P.; Zelenkofske, S. L.; Huang, Z.; Sailstad, J.; Armstrong, P. W.; Steg, P. G.; Bode, C.; Becker, R. C.; Alexander, J. H.; Adkinson, N. F.; Levinson, A. I., Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. Journal of Allergy and Clinical Immunology 2016, 138 (6), 1712-1715.
17. Duarte, I.; Temtem, M.; Gil, M.; Gaspar, F., Overcoming poor bioavailability through amorphous solid dispersions. Ind Pharm 2011, 30, 4-6.
18. Uchino, T.; Yasuno, N.; Yanagihara, Y.; Suzuki, H., Solid dispersion of spironolactone with porous silica prepared by the solvent method. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2007, 62 (8), 599-603.
19. Beg, S.; Swain, S.; Rizwan, M.; Irfanuddin, M.; Shobha Malini, D., Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Current Drug Delivery 2011, 8 (6), 691-702.
20. Im, K.; Ravi, A.; Kumar, D.; Kuttan, R.; Maliakel, B., An enhanced bioavailable formulation of curcumin using fenugreek-derived soluble dietary fibre. Journal of Functional Foods 2012, 4 (1), 348-357.
21. Shi, L.; Zhou, J.; Guo, J.; Gladden, I.; Kong, L., Starch inclusion complex for the encapsulation and controlled release of bioactive guest compounds. Carbohydrate Polymers 2021, 274, 118596.
22. Zhou, Y.; Li, H.; Yang, Y.-W., Controlled drug delivery systems based on calixarenes. Chinese Chemical Letters 2015, 26 (7), 825-828.
23. Polyakov, N. E.; Kispert, L. D., Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydrate polymers 2015, 128, 207-219.
24. Souto, E. B., Patenting nanomedicines: legal aspects, intellectual property and grant opportunities. Springer Science & Business Media: 2012.
25. Al-Marzouqi, A. H.; Elwy, H. M.; Shehadi, I.; Adem, A., Physicochemical properties of antifungal drug–cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. Journal of pharmaceutical and biomedical analysis 2009, 49 (2), 227-233.
26. Carrier, R. L.; Miller, L. A.; Ahmed, I., The utility of cyclodextrins for enhancing oral bioavailability. Journal of Controlled Release 2007, 123 (2), 78-99.
27. Huang, G.; Huang, H., Application of hyaluronic acid as carriers in drug delivery. Drug Delivery 2018, 25 (1), 766-772.
28. Cho, H.-J., Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. Journal of Pharmaceutical Investigation 2020, 50 (2), 115-129.
29. Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y., Recent advantage of hyaluronic acid for anti-cancer application: a review of “3S” transition approach. Carbohydrate Polymers 2020, 238, 116204.

